Abstract
This study assessed the relationships between maternal systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) and risk for autism spectrum disorders (ASDs) in offspring. Seven observational studies, including 25,005 ASD cases and 4,543,321 participants, were included for meta-analysis. Pooled results by using random-effects models suggested that maternal RA was associated with an increased risk for ASDs [odds ratio (OR) 1.39, 95% confidence interval (CI) 1.16–1.67], while maternal SLE was associated with an increased risk for ASDs only in western population (OR 1.91, 95% CI 1.02–3.57). Further study is warranted to confirm these results.
Similar content being viewed by others
Introduction
Autism spectrum disorders (ASDs) are complex neurodevelopmental disorders characterized by impairments in social communication, as well as restrictive and repetitive patterns of behaviors and interests (Lai et al. 2014). ASDs include infantile autism, Asperger’s syndrome, childhood disintegrative disorder, and pervasive developmental disorders not otherwise specified (American Psychiatric Association 2013). In 2010, one in 132 children globally was affected by ASD, with prevalence increasing significantly over the past decades (Baxter et al. 2015). Genetic and environmental factors both play roles in the pathogenesis of ASDs (Hertz-Picciotto et al. 2006). Maternal factors, such as prenatal obesity (Wang et al. 2016), prenatal diabetes (Xu et al. 2014), advanced age at delivery (Sandin et al. 2012), and caesarian section (Curran et al. 2015), among various environmental factors, have been suggested to be associated with increased risk for ASDs in offspring. Identification of these modifiable risk factors is of great significance for the primary prevention of ASDs.
Autoimmune diseases, which represent a heterogeneous group of disorders that afflict specific target organs or multiple organ systems, are chronic conditions initiated by the loss of immunological tolerance to selfantigens (Anaya et al. 2015). Systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) are two common autoimmune diseases, both of which have been associated with increased risk for several adverse outcomes such as spontaneous abortion, stillbirth, preterm birth, infants small for gestational age and low birth weight (Gleicher 2014; Lin et al. 2010). Although several studies have explored the relationship between maternal SLE and RA with ASDs among children, the conclusions have been inconclusive. Further, these associations have not been systematically reviewed. Therefore, we performed this study to systematically assess the association between maternal SLE and RA during pregnancy and the risk for ASDs in offspring.
Methods
Search Strategy
The present study adhered to the guidelines for meta-analysis of observational studies in epidemiology (Stroup et al. 2000). The PubMed, EMBASE, Web of Science, and Cochrane Library databases were searched up to March 20, 2019, for relevant observational studies investigating the association between maternal SLE or RA and risk for ASDs in offspring. In addition, Google Scholar was also searched, and the reference lists of all retrieved articles were manually scanned. The search terms used were “(Autoimmune diseases OR Systemic lupus erythematosus OR Rheumatoid arthritis) AND Autism”. No language restrictions were applied.
Study Selection Criteria
Observational studies that described an association between SLE or RA and risk for ASDs in offspring, and reported the risk estimates, including odds ratio (OR), relative risk (RR) or hazard ratio (HR) with corresponding 95% confidence interval (CI), or sufficient data to calculate them, were included. Letters, case reports, conference abstracts, reviews, and animal studies were excluded.
Data Extraction and Quality Assessment
Information extracted from selected studies included: name of the first author; publication year; study design; study location; characteristics of participants (sex and age); number of cases and participants; the RRs or ORs with corresponding 95% CIs; and confounding factors that were adjusted for in the analysis.
The Newcastle–Ottawa Scale (NOS) (Wells GA 2011) was used to assess study quality. A nine points scale was used to grade the included studies. The study would be regarded as low, moderate, and high quality when it scored 0–3, 4–6, and 7–9, respectively.
Data Analysis
OR was used as a measure of the association across studies. HR and RR were directly considered as OR, considering that the incidence of ASDs is rarely low. For two studies (Croen et al. 2019; Mouridsen et al. 2007), the OR values and corresponding 95% CIs were calculated using the original data from the case and control groups. Heterogeneity was assessed using the Cochrane Q statistic (significance level at P < 0.10) and the I2 statistic (Higgins and Thompson 2002; Higgins et al. 2003). The DerSimonian and Laird random-effects model (Mantel 1959) was applied to pool the results. Sensitivity analysis was conducted to explore the potential influence of each individual study on the pooled results. As the cultures, lifestyles, diets, and levels of medical services which may impact the health conditions of women varied between western populations and Asian populations, subgroup analyses according to study location (Western/Asian) were conducted. Meanwhile, subgroup analyses were also performed according to study design (cohort/case–control). Publication bias was assessed using the Begg’s test (Begg and Mazumdar 1994) and Egger’s test (Egger et al. 1997). STATA version 11.0 (StataCorp, College Station, TX, USA) was used for data analysis.
Results
Literature Search
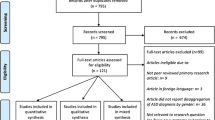
Figure 1 depicts the study selection flow. The database search yielded a total of 1161 articles, of which 340 duplicates were removed. By screening of titles and abstracts, 821 articles that were not observational studies or were animal studies, letters, case reports, and reviews, were excluded. Two full-text articles (Comi et al. 1999; Croen et al. 2005) did not provide sufficient data for meta-analysis and were excluded. Finally, seven observational studies (Atladottir et al. 2009; Croen et al. 2019; Keil et al. 2010; Mouridsen et al. 2007; Rom et al. 2018; Tsai et al. 2017; Vinet et al. 2015) were included, with four studies (Croen et al. 2019; Keil et al. 2010; Tsai et al. 2017; Vinet et al. 2015) reporting on SLE and five studies (Atladottir et al. 2009; Croen et al. 2019; Mouridsen et al. 2007; Rom et al. 2018; Tsai et al. 2017) reporting on RA.
Study Characteristics
The characteristics of the included studies are listed in Table 1. A total of 25,005 ASD cases and 4,543,321 participants were included in the analysis. There were four cohort studies (Atladottir et al. 2009; Rom et al. 2018; Tsai et al. 2017; Vinet et al. 2015) and three case–control studies (Croen et al. 2019; Keil et al. 2010; Mouridsen et al. 2007), which were conducted in Denmark (Atladottir et al. 2009; Mouridsen et al. 2007; Rom et al. 2018), China (Tsai et al. 2017), Canada (Vinet et al. 2015), United States (Croen et al. 2019) and Sweden (Keil et al. 2010), respectively. Information regarding maternal SLE, RA, and ASD in offspring was generally obtained from a medical register or health database. The mean NOS score (standard deviation) was 8.29 (0.76), there were no outliers of the NOS scores. More information could be found in Table 1.
Maternal SLE and the Risk for ASD in Offspring
Four studies (Croen et al. 2019; Keil et al. 2010; Tsai et al. 2017; Vinet et al. 2015) reported an association between maternal SLE and the risk for ASD in offspring. The random-effects model was used to pool the results (Pheterogeneity = 0.145; I2 = 44.5%). Overall, the meta-analysis suggested that maternal SLE was not associated with an increased risk for ASD in offspring (overall OR 1.32, 95% CI 0.64–2.72). Publication bias according to Begg’s and Egger’s tests was not evident (both P > 0.05) (Fig. 2).
A forest plot of the association between maternal SLE and risk of ASDs in offspring. Random-effects model was used to pool the overall odds ratios (ORs) and 95% confidence intervals (CIs). The diamond represents the pooled OR and, the squares and the horizontal lines respectively represent the RR and 95% CI of each individual study
Subgroup and sensitivity analysis both indicated that in western countries, there was a significant association between mothers with SLE and increased risk for ASDs in children (OR 1.91, 95% CI 1.02–3.57), and no statistically significant heterogeneity observed among these studies (Croen et al. 2019; Keil et al. 2010; Vinet et al. 2015) (Pheterogeneity = 0.374, I2 = 0.0%) (Table 2).
Maternal RA and the Risk for ASD in Offspring
Five studies (Atladottir et al. 2009; Croen et al. 2019; Mouridsen et al. 2007; Rom et al. 2018; Tsai et al. 2017) reported an association between maternal RA and risk for ASDs in offspring. Overall, maternal RA was associated with an increased risk for ASDs in offspring (overall OR 1.39, 95% CI 1.16–1.67). There was no statistically significant heterogeneity across studies (Pheterogeneity = 0.617, I2 = 0.0%), and no publication bias was detected using the Begg’s and Egger’s tests (all P > 0.05) (Fig. 3).
A forest plot of the association between maternal RA and risk of ASDs in offspring. Random-effects model was used to pool the overall odds ratios (ORs) and 95% confidence intervals (CIs). The diamond represents the pooled OR and, the squares and the horizontal lines respectively represent the RR and 95% CI of each individual study
Subgroup analysis according to study location and study design revealed that the pooled results in studies conducted in the western countries (OR 1.39, 95% CI 1.15–1.68) and in cohort studies (OR 1.38, 95% CI 1.14–1.67) were similar to the overall pooled results (Table 2). The sensitivity analysis presented generally robust results.
Discussion
To the best of our knowledge, this was the first meta-analysis of observational studies to assess the associations between maternal SLE, RA and the risk for ASDs in offspring. Based on seven included studies with more than 4.5 million participants, findings of the study suggested that maternal RA was associated with a 39% increased risk for ASDs in offspring. However, an increased risk was only found in children born to mothers with SLE from western countries based on three studies and 42,717 participants.
Possible Mechanisms
There were several plausible mechanisms to explain the possible relationship between SLE and RA to ASDs. First, autoimmune disease and ASDs may share common genetic paths. Several susceptibility alleles for autoimmune diseases have been found in offspring with ASDs and their mothers, including the human leukocyte antigen (HLA)-DRB1*04A (Fries et al. 2002; Johnson et al. 2009) and C4B null allele (Mostafa and Shehab 2010). Other susceptibility genes that may have important roles in regulating the developing immune system include PTEN, MET, and RELN (Gesundheit et al. 2013; Wu et al. 2015). Nevertheless, HLA-DRB1 alleles were not associated with ASDs for Chinese participants (Chien et al. 2012), while PTEN, MET, and RELN were more often found to be associated with ASDs in western populations than that in Asian ethnic groups. This may partially explain why the association between SLE and ASDs was only detected for western countries, but not for the Chinese.
Second, there exists immune mediators which may mediate the relationship between autoimmune disease and ASDs, such as cytokines and autoantibodies. Women with autoimmune disease exhibit high levels of cytokines and autoantibodies, which may reach the fetal circulation (Wojcik et al. 2017). Meanwhile, animal models have suggested that in utero exposure to these immune mediators affects fetal brain development and induces behavioral anomalies in offspring (Brimberg et al. 2013; Smith et al. 2007).
Third, immunoglobulin G (IgG) is the most prevalent antibody isotype in the human circulation that can cross the placental barrier and blood–brain barrier. Anti-SSA/Ro, anti-La/SS-B and anti-CCP autoantibodies, which cause SLE and RA, all belong to the IgG class (Fox-Edmiston and Van de Water 2015; Yoshimi et al. 2012). Moreover, pregnant women with autoimmune disease are prone to infections which may lead to increases in IgG levels (Wu et al. 2017). Previous studies indicated that children with ASDs were had elevated IgG levels compared with typically developing control children (Enstrom et al. 2009). It is likely that some types of IgG may pass through the placenta of women during pregnancy and impact the brain development of fetuses, which may eventually lead to ASDs.
Limitations
Several potential limitations of this meta-analysis warrant consideration. First, only 7 studies were included in the analysis, with four studies (Atladottir et al. 2009; Croen et al. 2019; Tsai et al. 2017; Vinet et al. 2015) involving SLE and 5 studies (Atladottir et al. 2009; Croen et al. 2019; Mouridsen et al. 2007; Rom et al. 2018; Tsai et al. 2017) addressing RA. The small number of eligible included studies may reduce the power to detect publication bias, although both the Begg’s and Egger’s tests indicated no evident publication bias. Moreover, the association between maternal SLE and ASDs among western population was based on only 42,717 participants, the relatively small sample size by comparison with the low incidence of ASDs may reduce the reliability of this finding. Further large-sample prospective cohort studies are needed to confirm the results of the present meta-analysis.
Second, although most included studies controlled several factors, such as maternal age at delivery, child’s birth year, sex, and residual confounders which may have interfered with the possible association of maternal SLE, RA and ASDs in offspring could not be ruled out. There are several known risk factors for ASDs, including maternal obesity (Wang et al. 2016), maternal diabetes (Xu et al. 2014), prenatal exposure to infection (Jiang et al. 2016) and fever (Brucato et al. 2017), there are evidence that these factors may also be associated with RA or SLE (Bender Ignacio et al. 2018; Dong et al. 2019; Feng et al. 2019; Liu et al. 2017). However, only one study adjusted for maternal diabetes (Vinet et al. 2015) and no studies controlled for maternal body weight status. Therefore, the association between maternal RA and ASD found in this meta-analysis may, in part, be due to these unadjusted risk factors for ASD rather than to autoimmunity alone.
Third, misclassification bias may exist in the identification of SLE, RA, and ASDs. For the diagnosis of ASDs, the Chinese sample used ICD-9 (Tsai et al. 2017), the Canadian sample used both ICD-9 and ICD-10 (Vinet et al. 2015), and the three Danish samples used the 8th to 10th versions of ICD (Atladottir et al. 2009; Mouridsen et al. 2007; Rom et al. 2018). The definition of the diagnostic entities of ASD had been improved considerably by the transition from ICD-8 to ICD-10 and become more inclusive (Lauritsen et al. 2010). Meanwhile, methods for ascertainment of SLE, RA also varied across studies. Pregnancy itself has a positive effect on the symptoms and signs of RA, with 75% of patients experiencing improvement or remission of arthritis during gestation (Ostensen and Villiger 2007). Mothers with SLE and RA diagnosed at baseline may be cured during follow-up and before pregnancy. In addition, information regarding ASDs was self-reported or obtained from medical records in invalidated registries in three case–control studies (Croen et al. 2019; Keil et al. 2010; Mouridsen et al. 2007). Due to the above reasons, there may have been an underestimation or exaggerated risk estimates if the extent of misclassification in two groups were different. Future studies are suggested to use the latest diagnostic criteria for the ascertainments and validation of both exposures and ASDs.
Fourth, the study population varied across studies, with six studies (Atladottir et al. 2009; Croen et al. 2019; Keil et al. 2010; Mouridsen et al. 2007; Rom et al. 2018; Vinet et al. 2015) conducted in western countries including Denmark, Sweden, USA, and Canada while one study (Tsai et al. 2017) involved an Asian population (China). Moreover, maternal race-ethnicity for ASD case group and control group was incomparable in three studies (Croen et al. 2019; Keil et al. 2010; Vinet et al. 2015), in which mothers were more or less likely to be white population for case group or for control group. Nevertheless, both ASD and SLE are known to have strong genetic links, with heritability indices of 0.85–0.92 for ASD (Miles 2011) and up to 0.66 for SLE (Lewis & Jawad 2017). The prevalence of ASD varies in the US by race, with an estimation of 7% and 22% higher in white children than that in black children and Hispanic children, respectively (Jon et al. 2018). Meanwhile, higher risk of developing SLE and severe manifestations has been observed in Asians, Africans, and other non-white groups than that in white Europeans (Lewis and Jawad 2017). As only one study controlled for race (Vinet et al. 2015), the reported risk of ASDs in relation to maternal SLE and RA could have been partially affected by the variation in study population and race of participants.
Finally, apart from four cohort studies (Atladottir et al. 2009; Rom et al. 2018; Tsai et al. 2017; Vinet et al. 2015), we also included three case–control studies (Croen et al. 2019; Keil et al. 2010; Mouridsen et al. 2007) that retrospectively collected information on exposure or confounding factors. Therefore, recall bias was inevitable. In addition, because we did not have access to individual patient-based data from the original studies, we failed to provide a more powerful and less-biased individual patient data-based meta-analysis.
Conclusion
In summary, there was an increased overall risk for ASDs in offspring born to mothers with RA and to mothers with SLE from western countries. Given the limited number of included studies, further well-designed prospective cohort studies including different ethnicities are needed to confirm the findings of this meta-analysis.
References
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author.
Anaya, J. M., Rojas-Villarraga, A., & García-Carrasco, M. (2015). The autoimmune tautology: From polyautoimmunity and familial autoimmunity to the autoimmune genes. Autoimmune Diseases. https://doi.org/10.1155/2012/297193.
Atladottir, H. O., et al. (2009). Association of family history of autoimmune diseases and autism spectrum disorders. Pediatrics,124, 687–694. https://doi.org/10.1542/peds.2008-2445.
Baxter, A. J., Brugha, T. S., Erskine, H. E., Scheurer, R. W., Vos, T., & Scott, J. G. (2015). The epidemiology and global burden of autism spectrum disorders. Psychological Medicine,45, 601–613. https://doi.org/10.1017/s003329171400172x.
Begg, C. B., & Mazumdar, M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics,50, 1088–1101.
Bender Ignacio, R. A., Madison, A. T., Moshiri, A., Weiss, N. S., & Mueller, B. A. (2018). A population-based study of perinatal infection risk in women with and without systemic lupus erythematosus and their infants. Paediatric and Perinatal Epidemiology,32, 81–89.
Brimberg, L., Sadiq, A., Gregersen, P. K., & Diamond, B. (2013). Brain-reactive IgG correlates with autoimmunity in mothers of a child with an autism spectrum disorder. Molecular Psychiatry,18, 1171–1177.
Brucato, M., et al. (2017). Prenatal exposure to fever is associated with autism spectrum disorder in the boston birth cohort. Autism Research,10, 1878–1890. https://doi.org/10.1002/aur.1841.
Chien, Y. L., et al. (2012). Association of HLA-DRB1 alleles and neuropsychological function in autism. Psychiatric Genetics,22, 46–49. https://doi.org/10.1097/YPG.0b013e32834915ae.
Comi, A. M., Zimmerman, A. W., Frye, V. H., Law, P. A., & Peeden, J. N. (1999). Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. Journal of Child Neurology,14, 388–394. https://doi.org/10.1177/088307389901400608.
Croen, L. A., Grether, J. K., Yoshida, C. K., Odouli, R., & Van de Water, J. (2005). Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: A case–control study. Archives of Pediatrics and Adolescent Medicine,159, 151–157. https://doi.org/10.1001/archpedi.159.2.151.
Croen, L. A., et al. (2019). Family history of immune conditions and autism spectrum and developmental disorders: Findings from the study to explore early development. Autism Research,12, 123–135. https://doi.org/10.1002/aur.1979.
Curran, E. A., et al. (2015). Research Review: Birth by caesarean section and development of autism spectrum disorder and attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Journal of Child Psychology and Psychiatry and Allied Disciplines,56, 500–508.
Dong, Y., et al. (2019). Risk of gestational diabetes mellitus in systemic lupus erythematosus pregnancy: A systematic review and meta-analysis. BMC Pregnancy Childbirth,19, 179.
Egger, M., Davey Smith, G., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ,315, 629–634.
Enstrom, A., et al. (2009). Increased IgG4 levels in children with autism disorder. Brain, Behavior, and Immunity,23, 389–395.
Feng, X., et al. (2019). Body mass index and the risk of rheumatoid arthritis: An updated dose-response meta-analysis. BioMed Research International. https://doi.org/10.1155/2019/3579081.
Fox-Edmiston, E., & Van de Water, J. (2015). Maternal anti-fetal brain IgG autoantibodies and autism spectrum disorder: Current knowledge and its implications for potential therapeutics. CNS Drugs,29, 715–724. https://doi.org/10.1007/s40263-015-0279-2.
Fries, J. F., et al. (2002). HLA–DRB1 genotype associations in 793 white patients from a rheumatoid arthritis inception cohort: Frequency, severity, and treatment bias. Arthritis & Rheumatology,46, 2320–2329.
Gesundheit, B., et al. (2013). Immunological and autoimmune considerations of autism spectrum disorders. Journal of Autoimmunity,44, 1–7.
Gleicher, N. (2014). Maternal autoimmunity and adverse pregnancy outcomes. Journal of Autoimmunity,50, 83–86. https://doi.org/10.1016/j.jaut.2013.12.009.
Hertz-Picciotto, I., Croen, L. A., Hansen, R., Jones, C. R., van de Water, J., & Pessah, I. N. (2006). The CHARGE study: An epidemiologic investigation of genetic and environmental factors contributing to autism. Environmental Health Perspectives,114, 1119.
Higgins, J. P., & Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Statistics in Medicine,21, 1539.
Higgins, J. P. T., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta-analyses. British Medical Journal,327, 557–560.
Jiang, H. Y., et al. (2016). Maternal infection during pregnancy and risk of autism spectrum disorders: A systematic review and meta-analysis. Brain, Behavior, and Immunity,58, 165–172. https://doi.org/10.1016/j.bbi.2016.06.005.
Johnson, W. G., et al. (2009). HLA-DR4 as a risk allele for autism acting in mothers of probands possibly during pregnancy. Archives of Pediatrics and Adolescent Medicine,163, 542–546.
Jon, B., et al. (2018). Prevalence of autism spectrum disorder among children aged 8 years—Autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR. Surveillance Summaries,67, 1–23.
Keil, A., et al. (2010). Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiology,21, 805–808. https://doi.org/10.1097/EDE.0b013e3181f26e3f.
Lai, M. C., Lombardo, M. V., & Baron-Cohen, S. (2014). Autism. Lancet,383, 896–910. https://doi.org/10.1016/s0140-6736(13)61539-1.
Lauritsen, M. B., et al. (2010). Validity of childhood autism in the Danish Psychiatric Central Register: Findings from a cohort sample born 1990-1999. Journal of Autism and Developmental Disorders,40, 139–148.
Lewis, M. J., & Jawad, A. S. (2017). The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology (Oxford),56, i67–i77.
Lin, H. C., Chen, S. F., Lin, H. C., & Chen, Y. H. (2010). Increased risk of adverse pregnancy outcomes in women with rheumatoid arthritis: A nationwide population-based study. Annals of the Rheumatic Diseases,69, 715–717. https://doi.org/10.1136/ard.2008.105262.
Liu, Y., Hazlewood, G. S., Kaplan, G. G., Eksteen, B., & Barnabe, C. (2017). Impact of obesity on remission and disease activity in rheumatoid arthritis: A systematic review and meta-analysis. Arthritis Care & Research (Hoboken),69, 157–165.
Mantel, N. (1959). Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute,22, 719–748.
Miles, J. H. (2011). Autism spectrum disorders—A genetics review. Genetics in Medicine,13, 278–294.
Mostafa, G. A., & Shehab, A. A. (2010). The link of C4B null allele to autism and to a family history of autoimmunity in Egyptian autistic children. Journal of Neuroimmunology,223, 115–119.
Mouridsen, S. E., Rich, B., Isager, T., & Nedergaard, N. J. (2007). Autoimmune diseases in parents of children with infantile autism: A case–control study. Developmental Medicine and Child Neurology,49, 429–432. https://doi.org/10.1111/j.1469-8749.2007.00429.x.
Ostensen, M., & Villiger, P. M. (2007). The remission of rheumatoid arthritis during pregnancy. Seminars in Immunopathology,29, 185–191.
Rom, A. L., Wu, C. S., Olsen, J., Jawaheer, D., Hetland, M. L., & Morch, L. S. (2018). Parental rheumatoid arthritis and autism spectrum disorders in offspring: A Danish Nationwide Cohort Study. Journal of the American Academy of Child and Adolescent Psychiatry,57(28–32), e1. https://doi.org/10.1016/j.jaac.2017.10.002.
Sandin, S., Hultman, C. M., Kolevzon, A., Gross, R., Maccabe, J. H., & Reichenberg, A. (2012). Advancing maternal age is associated with increasing risk for autism: A review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry,51, 477.e1–486.e1.
Smith, S. E., Li, J., Garbett, K., Mirnics, K., & Patterson, P. H. (2007). Maternal immune activation alters fetal brain development through interleukin-6. Journal of Neuroscience,27, 10695–10702.
Stroup, D. F., et al. (2000). Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA,283, 2008–2012.
Tsai, P. H., et al. (2017). Risk of autism spectrum disorder in children born to mothers with systemic lupus erythematosus and rheumatoid arthritis in Taiwan. Joint Bone Spine. https://doi.org/10.1016/j.jbspin.2017.11.005.
Vinet, E., et al. (2015). Increased risk of autism spectrum disorders in children born to women with systemic lupus erythematosus: Results from a large population-based cohort. Arthritis & Rheumatology,67, 3201–3208. https://doi.org/10.1002/art.39320.
Wang, Y., Tang, S., Xu, S., Weng, S., & Liu, Z. (2016). Maternal Body Mass Index and Risk of Autism Spectrum Disorders in Offspring: A Meta-analysis. Scientific Reports,6, 34248.
Wells, G.A., Shea, B., O’Connell, D., Peterson, J., Welch, V., Losos, M., Tugwell, P. (2011). The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.
Wojcik, S., et al. (2017). Risk of autism spectrum disorders in children born to mothers with rheumatoid arthritis: A systematic literature review. Arthritis Care & Research,69(12), 1926–1931.
Wu, S., et al. (2015). Family history of autoimmune diseases is associated with an increased risk of autism in children: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews,55, 322.
Wu, L., et al. (2017). T cell subsets and immunoglobulin G levels are associated with the infection status of systemic lupus erythematosus patients. Brazilian Journal of Medical and Biological Research,51, e4547. https://doi.org/10.1590/1414-431x20154547.
Xu, G., Jing, J., Bowers, K., Liu, B., & Bao, W. (2014). Maternal diabetes and the risk of autism spectrum disorders in the offspring: A systematic review and meta-analysis. Journal of Autism and Developmental Disorders,44, 766–775.
Yoshimi, R., Ueda, A., Ozato, K., & Ishigatsubo, Y. (2012). Clinical and pathological roles of Ro/SSA autoantibody system. Clinical and Developmental Immunology. https://doi.org/10.1155/2012/606195.
Author information
Authors and Affiliations
Contributions
YW conceived of the study, participated in its design and coordination and drafted the manuscript; ST participated in the design and interpretation of the data; XD participated in the design and coordination of the study and performed the measurement; ZZ, ST participated in the design of the study and performed the statistical analysis; ZZ, YW conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, Z., Tang, S., Deng, X. et al. Maternal Systemic Lupus Erythematosus, Rheumatoid Arthritis, and Risk for Autism Spectrum Disorders in Offspring: A Meta-analysis. J Autism Dev Disord 50, 2852–2859 (2020). https://doi.org/10.1007/s10803-020-04400-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-020-04400-y